MYONEX INSIGHTS
Ancillary Wishlists & The ‘one product suits all’ Conundrum
Posted on 05/06/22All around the world today, sitting in Pharmaceutical, Biotechs and Clinical Research organizations, like-minded and well-intentioned professionals within the life science industry are looking to enhance world healthcare and support the delivery of new medications and products for patients around the world.
The scene is set, the IP/Drug intended for the trial has been prepared, patient enrolment is both successful and underway, initial sites are prepared for SIV’s and Principal Investigators are eagerly awaiting to start and contribute to improving healthcare for patients around the world. One final step to take part of within the clinical trial to go; consisting of drawing up lists of products/equipment required for use across globally enrolled and soon to be activated sites. The light is at the end of the tunnel!
Importantly, a large proportion of the world’s largest pharmaceutical organizations that conduct regular, global clinical trials are based or have headquarters within the US. A key aspect of sourcing clinical trial equipment and supplies as a result puts significant emphasis on the supply of equipment available locally, in this case, the US. Commonly, when it comes to clinical trials and ancillary supply, it is not unheard of for the supply of ancillaries, devices, and consumable products to be thought of at the very last hurdle, or at the very end stages of a clinical trial design. All the hard work has taken place months ago, from drug development, regulatory and feasibility/financial budgeting processes, and site/patient identification. Once these aspects are approved, regularly, the pressure is now on for pharmaceutical providers to begin the trial, which means quickly sourcing and supplying clinical sites with equipment. As a result, a widespread and comprehensive list of medical devices and consumables are drawn up in what can be described as an ‘Ancillary Wishlist’ with all the items a clinical site could desire. What is that I hear you say? “All I need is a sharps bin, which can’t be difficult for my global (US&UK) trial, one product will do, so a red one will do just fine in this list. Easy right?”
Let’s take a step back with the scenario above and understand the needs of the clinical team. The team requires the provision of standard medical sharps bins for the safe disposal of sharps used within the trial for medicine/drug delivery to the patient. When looking into the supply side of sharps bins in the global study (for use in the US and UK in this example), it’s important to note that in this case, one size does not fit all, or in this case ‘a red one’ will not ‘just do fine’. What is meant by this is that there are different regulations, categorizations and types of products which are used in different regions around the world which have different local requirements.
For example, used/contaminated sharps waste in the US explained by the Environmental Protection Agency (EPA) as Medical Waste Guidance can be categorized as ‘Medical Waste, specifically that of Infectious Waste’ which has specific use for any waste that could cause an infection in humans, like blood, human tissue or anything contaminated with body fluids (non-cytotoxic). In this case, sharps for use in a clinical trial which have been used to deliver non-cytotoxic medicines, would need to be color coordinated with a white/clear lid and red sharps bin:
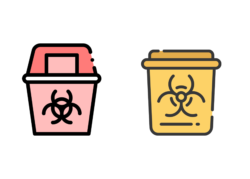
When looking to the UK’s market for contaminated sharps waste, this can be classified ensuring compliance with the ‘Safe Management of Healthcare Waste’ guidance published via the UK Department of Health and in addition to the UK Governments website similarly referring to Sharps waste as ‘Hazardous Waste, specifically that of Sharps Related Waste, categorized as Other Medically Contaminated or Clinical Highly Infectious Waste’ which as a result must have clear color-coded identifiers. In this case, sharps for use in a clinical trial which have been used to deliver non-cytotoxic medicines, would need to be color coordinated with a yellow lid and yellow sharps bin shown above.
At Myonex, our mission is to provide superior clinical trial supply solutions to support our customers within the clinical trials marketplace. We focus on our customers and work tirelessly to provide fast and efficiently the best solutions to the trial and offer ways to provide product expertise, guidance, and best practice application in trials. By keeping our customers at the forefront of our minds throughout the sourcing, supply and end of a study, we are playing a small part in the bigger healthcare picture within clinical trials.
 Mike Riley
Mike Riley
Procurement Manager, Clinical Trial Equipment and Supplies

